HRZE and the gut microbiome
 This tutorial is a walkthrough of the data analysis from:
This tutorial is a walkthrough of the data analysis from:
Antibiotic treatment for Tuberculosis induces a profound dysbiosis of the microbiome that persists long after therapy is completed
Scientific Reports 7, Article number: 10767 (2017)
doi: 10.1038/s41598-017-10346-6
It shows how to take microbiome data and reproduce the figures from this paper.
Part 1: Import 16S uparse output into R
I probably have more packages here than I are actually needed to generate the figures in this tutorial, but I have never had a problem loading all of them in this order. One exception is DESeq, which needs to be loaded after the Phyloseq object is constructed.
setwd("~/Desktop/uparse_july/")
rm(list=ls())
library(plyr);library(ggtree);library(phyloseq);library(ggplot2);library(scales);library(grid)
library(Hmisc);library(gridExtra);library(scales);library(stringr);library(logistf)
library(coxphf);library(reshape2);library(ifultools);library(car);library(vegan)
library(gdata);library(chron);library(data.table);library(tidyr) #imports tibble
library(ggplot2);library(yingtools2);library(gridExtra);library(lubridate);library(dplyr)
library("pheatmap");library("RColorBrewer");library("genefilter");library(ggthemes)
Give priority to specific functions for annoying namespace reasons
select <- dplyr::select
summarize <- dplyr::summarize
rownames_to_column <- tibble::rownames_to_column
Necessary uparse pipeline output files for downstream 16S data analysis
These are the four files generated from a slightly modified version of the uparse pipeline. The only truly unique file here is the tax.file (repset.fasta.blastn.refseq_rna.txt), which contains information about the top 30 BLASTn hits for each OTU in the seq.file (total.5.repset.fasta). The biom.file (total.8.otu-tax.biom) is a type of data frame where the columns correspond to people and the rows correspond to each OTU from the seq.file. Additionally, this file can hold metadata about either the rows (OTUs) or columns (the people), and in this case, the taxonomy metadata corresponding to the GreenGenes taxonomy names. Finally, the tree.file is generated from a Qiime python script called make_phylogeny.py, which represents true evolutionary distance between these OTU sequences (i.e., the seq.file). These four files generated from uparse, qiime, and blast contain all of the information one needs to accurately analyze microbiome data and generate the types of plots in this tutorial. I make use of many commands from the yingtools2 R package, written by Ying Taur at MSKCC.
#edit the $PATH to these files
biom.file <- "~/Desktop/uparse_july/total.8.otu-tax.biom"
seq.file <- "~/Desktop/uparse_july/total.5.repset.fasta"
tax.file <- "~/Desktop/uparse_july/total.5.repset.fasta.blastn.refseq_rna.txt"
tree.file <- "~/Desktop/uparse_july/total.10.tree"
biom <- import_biom(biom.file)
seq <- import_qiime(refseqfilename=seq.file)
tree <- read.tree(text=scan(tree.file,what=character(),quiet=TRUE))
#make tax object with BLAST names (skip this step to assign GreenGenes names instead)
tax <- yingtools2::read.blastn.file(tax.file) %>% set.tax()
Import and setup sample data (metadata for each person in the study)
data.file <- "TBRU_Metadata_May2017.csv"
data <- read.csv(data.file,na.strings=c("N/A","99999")) %>% mutate(sample=gsub("\\-",".",sample))
samp <- data %>% data.frame()
#fix some name issues in the biom file to make sample_names(biom) consistent
sample_names(biom) <- gsub("..pool749","",sample_names(biom))
sample_names(biom) <- gsub("\034","",sample_names(biom))
Merge all data together to create the final phyloseq object
phy <- merge_phyloseq(biom,seq,tree)
tax_table(phy) <- tax
sample_data(phy) <- samp %>% set.samp()
Part 2: Subset samples and run DESeq data normalization
#IGRA positive, only community cohort vs treatment
phyIGRA_pos_treatment <- subset_samples(phy,!is.na(IGRA) & IGRA!="negative") #can change this first variable to increase contorl sample size by including all IGRA- people, change "negative" to ""
phyIGRA_pos_treatment <- subset_samples(phyIGRA_pos_treatment,!is.na(IGRA) & IGRA!="")
phyIGRA_pos_treatment <- subset_samples(phyIGRA_pos_treatment,!is.na(TB_status) & TB_status!="cured")
phyIGRA_pos_treatment <- subset_samples(phyIGRA_pos_treatment,!is.na(TB_status) & TB_status!="")
phyIGRA_pos_treatment <- subset_samples(phyIGRA_pos_treatment,!is.na(Group6_TB_category) & Group6_TB_category!="family_contact")
phyIGRA_pos_treatment <- subset_samples(phyIGRA_pos_treatment,age < 33)
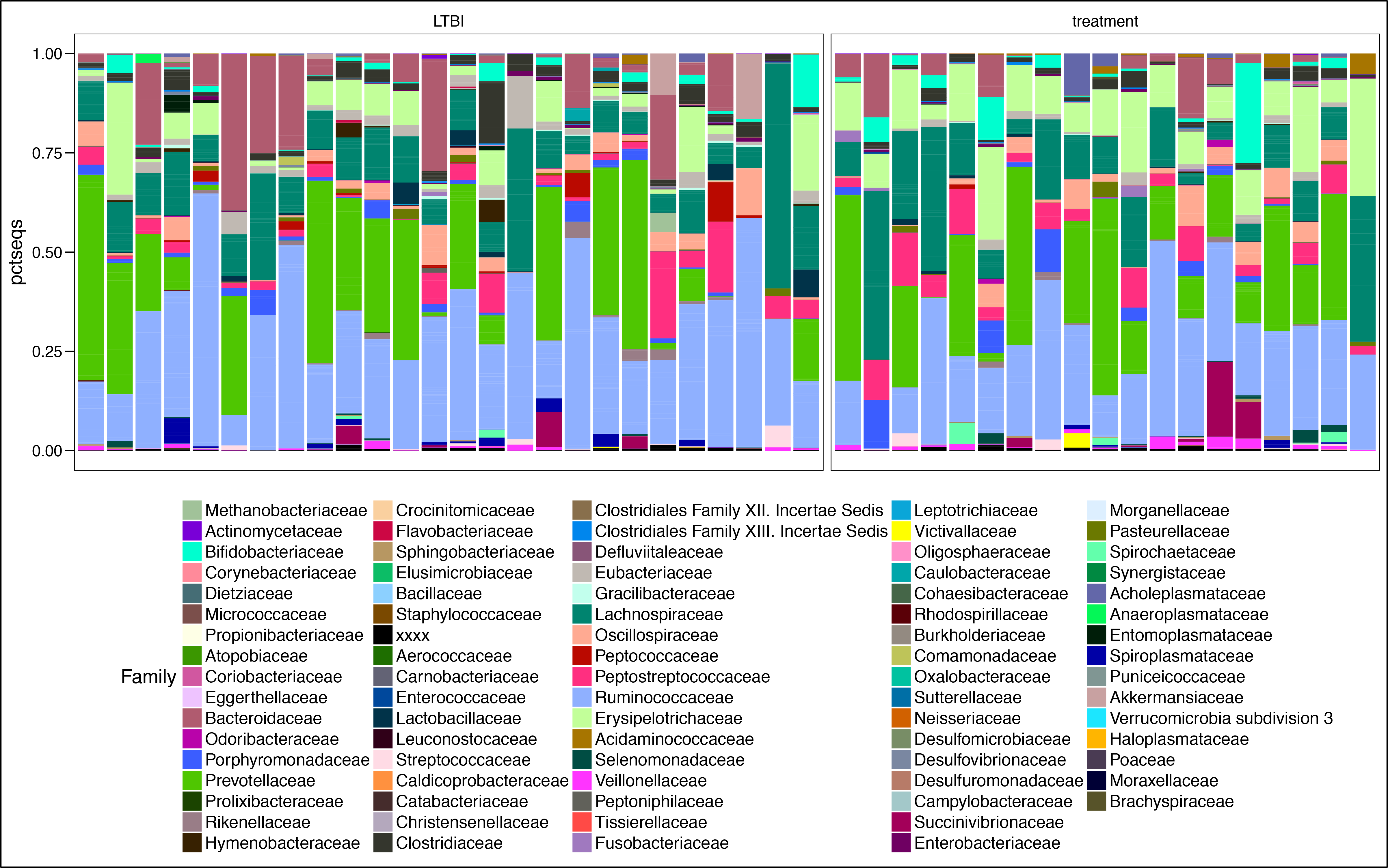
View the Family-level distribution of taxa between the two groups
assignCols <- function(names,selection) {
mymap<-list()
availableCols <-c("#000000", "#FFFF00", "#1CE6FF", "#FF34FF", "#FF4A46", "#008941", "#006FA6", "#A30059",
"#FFDBE5", "#7A4900", "#0000A6", "#63FFAC", "#B79762", "#004D43", "#8FB0FF", "#997D87",
"#5A0007", "#809693", "#FEFFE6", "#1B4400", "#4FC601", "#3B5DFF", "#4A3B53", "#FF2F80",
"#61615A", "#BA0900", "#6B7900", "#00C2A0", "#FFAA92", "#FF90C9", "#B903AA", "#D16100",
"#DDEFFF", "#000035", "#7B4F4B", "#A1C299", "#300018", "#0AA6D8", "#013349", "#00846F",
"#372101", "#FFB500", "#C2FFED", "#A079BF", "#CC0744", "#C0B9B2", "#C2FF99", "#001E09",
"#00489C", "#6F0062", "#0CBD66", "#EEC3FF", "#456D75", "#B77B68", "#7A87A1", "#788D66",
"#885578", "#FAD09F", "#FF8A9A", "#D157A0", "#BEC459", "#456648", "#0086ED", "#886F4C",
"#34362D", "#B4A8BD", "#00A6AA", "#452C2C", "#636375", "#A3C8C9", "#FF913F", "#938A81",
"#575329", "#00FECF", "#B05B6F", "#8CD0FF", "#3B9700", "#04F757", "#C8A1A1", "#1E6E00",
"#7900D7", "#A77500", "#6367A9", "#A05837", "#6B002C", "#772600", "#D790FF", "#9B9700",
"#549E79", "#FFF69F", "#201625", "#72418F", "#BC23FF", "#99ADC0", "#3A2465", "#922329",
"#5B4534", "#FDE8DC", "#404E55", "#0089A3", "#CB7E98", "#A4E804", "#324E72", "#6A3A4C",
"#83AB58", "#001C1E", "#D1F7CE", "#004B28", "#C8D0F6", "#A3A489", "#806C66", "#222800",
"#BF5650", "#E83000", "#66796D", "#DA007C", "#FF1A59", "#8ADBB4", "#1E0200", "#5B4E51",
"#C895C5", "#320033", "#FF6832", "#66E1D3", "#CFCDAC", "#D0AC94", "#7ED379", "#012C58")
mymap[[1]]<-rev(availableCols[match(intersect(names,selection),names)])
mymap[[2]]<-intersect(names,selection)
mymap
};
#function to make a tax plot faceted by whatever variable
plot.tax <- function(phyloseq, variable){
t <- get.otu.melt(phyIGRA_pos_treatment) %>% arrange(Kingdom, Phylum, Class, Order, Family, Genus, Class) %>%
mutate(TB_status = factor(TB_status,levels = unique(TB_status))) %>% group_by(sample) %>%
arrange(TB_status) %>% mutate(cum.pct = cumsum(pctseqs),
y.text = (cum.pct + c(0, cum.pct[-length(cum.pct)]))/2) %>%
ungroup() %>% dplyr::select(-cum.pct)
g <- ggplot() + geom_bar(data = t, aes_string(x = "sample",
y = "pctseqs", fill = "Order"), stat = "identity",position = "fill") +
theme(legend.position = "bottom") + facet_grid(~TB_status,scales="free",space="free")
g
return(g)
}
t <- get.otu.melt(phyIGRA_pos_treatment) %>% arrange(Kingdom, Phylum, Class, Order, Family, Genus, Class) %>%
mutate(TB_status = factor(TB_status,levels = unique(TB_status))) %>% group_by(sample) %>%
arrange(TB_status) %>% mutate(cum.pct = cumsum(pctseqs),
y.text = (cum.pct + c(0, cum.pct[-length(cum.pct)]))/2) %>%
ungroup() %>% dplyr::select(-cum.pct) %>% as.data.frame()
#show the Family level in the plot
mycol <- assignCols(unique(t$Family),unique(t$Family))
barplot <- ggplot() + geom_bar(data = t, aes_string(x = "sample", y = "pctseqs", fill = "Family"),stat = "identity",position = "fill") +
facet_grid(~TB_status,scales="free",space="free") +
theme(legend.position = "bottom", axis.title.x=element_blank(),axis.text.x=element_blank(),axis.ticks.x=element_blank()) +
theme(legend.text = element_text(size = 14)) +
scale_fill_manual(values = mycol[[1]],breaks=mycol[[2]])
barplot
Load the DESeq2 package, make taxa names more friendly
Downstream plotting of log2 differential abundance data from the DESeq output is made easier when the Species name and the specific OTU number are merged, since multiple OTUs can have the same name
library("DESeq2")
phy_DESeq <- phyIGRA_pos_treatment
t <- get.tax(phy_DESeq) %>% mutate(PhySpec=paste(Species,otu))
taxa_names(phy_DESeq) <- t$PhySpec
taxa_names(phy_DESeq) <- gsub(" ","_",taxa_names(phy_DESeq))
taxa_names(phy_DESeq) <- gsub("=","_",taxa_names(phy_DESeq))
taxa_names(phy_DESeq) <- gsub(";","_",taxa_names(phy_DESeq))
Check the variance of OTU abundances
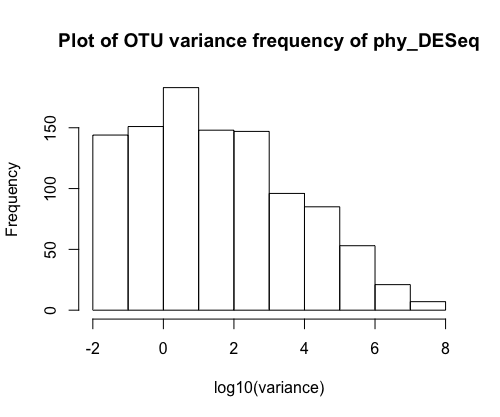
This plot tells you that most of the OTUs do not vary much from the raw sequencing data (this is a property of zero inflated data), but some have rather high variance. DESeq will attempt to normalize this variance with respect to a particular sample variable.
hist(log10(apply(otu_table(phy_DESeq), 1, var)), xlab = "log10(variance)", main = "A large fraction of OTUs have very low variance")
Convert the phyloseq object to DESeq object, normalize with respect to TB status, and plot the results
dig <- phyloseq_to_deseq2(phy_DESeq, ~ TB_status) #replace this with any sample variable(s)
#calculate geometric mean
gm_mean <- function(x, na.rm=TRUE){
exp(sum(log(x[x > 0]), na.rm=na.rm) / length(x))
}
geoMeans <- apply(counts(dig), 1, gm_mean)
dig <- estimateSizeFactors(dig, geoMeans = geoMeans)
dig <- estimateDispersions(dig)
dig <- DESeq(dig,fitType= "local")
res <- results(dig)
#res$pfdr <- p.adjust(res$pvalue,method="fdr")
res <- res[order(res$padj, na.last=NA), ]
alpha <- 0.05
sigtab <- res[(res$padj < alpha), ]
sigtab <- cbind(as(sigtab, "data.frame"), as(tax_table(phy_DESeq)[rownames(sigtab), ], "matrix"))
head(sigtab) #view the data
posigtab <- sigtab[sigtab[, "log2FoldChange"] > 1, ]
posigtab <- posigtab[, c("baseMean", "log2FoldChange", "lfcSE", "padj", "Phylum", "Class", "Family", "Genus")]
library("ggplot2")
theme_set(theme_bw())
sigtabgen <- subset(sigtab, !is.na(Genus))
sigtabgen <- subset(sigtab, !is.na(Phylum))
#Reorder the data to display the genus
x <- tapply(sigtabgen$log2FoldChange, sigtabgen$Genus, function(x) max(x))
x <- sort(x, TRUE)
sigtabgen$Genus <- factor(as.character(sigtabgen$Genus), levels=names(x))
phylumcolors <- c("hotpink","brown","lightblue","purple")
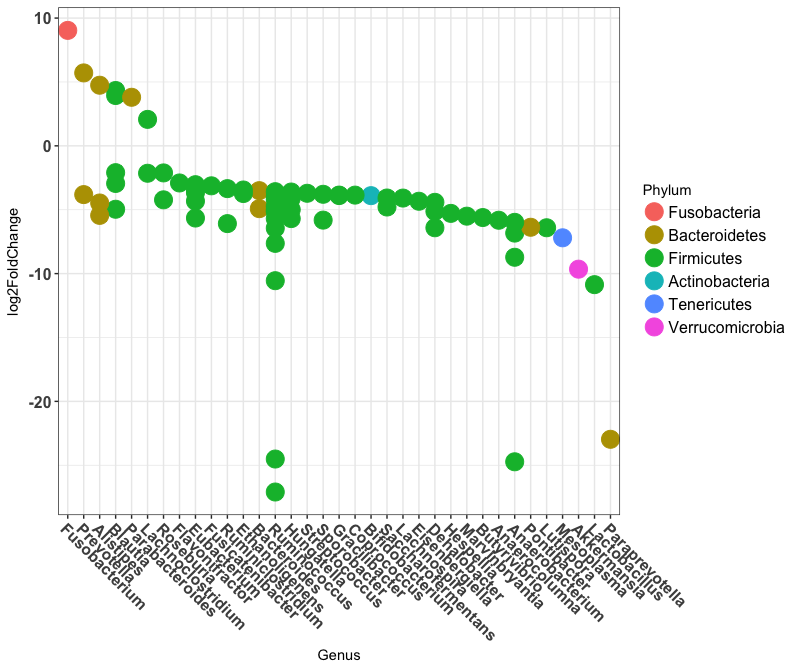
ggplot(sigtabgen, aes(x = Genus, y = log2FoldChange, color = Phylum)) + geom_point(size = 6) +
theme(axis.text.x = element_text(angle = -45, hjust = 0, vjust = 1)) +
theme(axis.text=element_text(size=12,face="bold"),legend.text=element_text(size=12))
This plot shows the log2 fold change between IGRA+ people and people on HRZE antibiotic treatment for Tuberculosis. Most of the OTUs fall below zero because they are depleted in people taking antibiotics. Some OTUs are increased however, potentially taking advantage of the altered ecosystem of the gut while taking antibiotics.
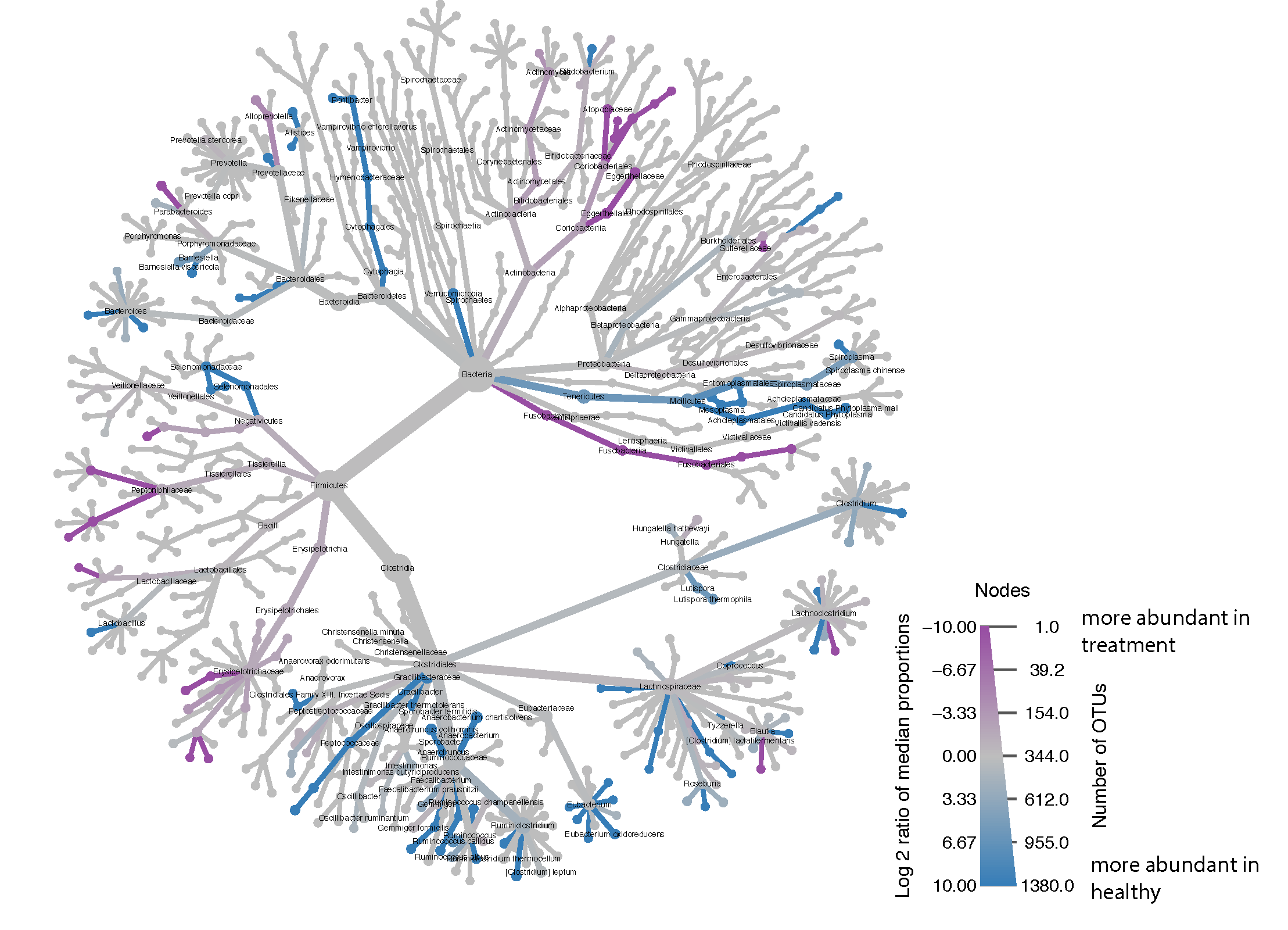
Part 3: Analyze data with LEfSe
LEfSe website: LEfSe
Check the $PATH in RStudio to make sure LEfSe Python scripts are there
system2("echo",args = "$PATH")
#RStudio should inherit the system path if it is opened at the command line with "open -a RStudio"
#acquire sample data from subsetted phyloseq object and set LEfSe parameters
phy.lefse <- phy_DESeq
results_folder <- "~/Desktop/uparse_july/"
class <- "TB_status"
subclass<-FALSE
subject<-"sample"
anova.alpha<-0.05 #this is the important p value
wilcoxon.alpha<-0.05
lda.cutoff<-3.0
wilcoxon.within.subclass <- TRUE
one.against.one <- T
mult.test.correction <- 0
make.lefse.plots <- FALSE
by_otus <- FALSE
#
sample.data <- phyloseq::sample_data(phy.lefse) %>% data.frame(stringsAsFactors = FALSE)
sample.data$sample <- rownames(sample.data)
#
keepvars <- c("sample","TB_status")
keepvars <- unique(keepvars[!is.na(keepvars)])
lefse.samp <- sample.data[, keepvars]
#
sample0 <- t(lefse.samp) %>% as.matrix()
colnames(sample0) <- sample0[1,]
sample0 <- as.data.frame(sample0)
#
data0 <- otu_table(phy.lefse) %>% as.data.frame()
data1 <- data0 %>% as.data.table(keep.rownames=T)
sample1 <- sample0 %>% as.data.table(keep.rownames=T)
common <- intersect(colnames(data1), colnames(sample1))
pre.lefse <- rbind(sample1, data1,fill=T) %>% t() %>% na.omit() %>% t()
#writes table for LEfSe
write.table(pre.lefse,file =paste(results_folder,"lefse.txt",sep=""),sep = "\t",row.names = FALSE,col.names = FALSE,quote = FALSE)
opt.class <- paste("-c", which(keepvars %in% class))
opt.subclass <- ifelse(is.na(subclass), "", paste("-s", which(keepvars %in%
subclass)))
opt.subject <- ifelse(is.na(subject), "", paste("-u", which(keepvars %in%
subject)))
format.command <- paste(paste("format_input.py ",results_folder,"lefse.txt ",results_folder,"lefse.in",sep=""),
opt.class, opt.subject, "-o 1000000")
format.command
#"format_input.py ~/Desktop/uparse_july/lefse.txt ~/Desktop/uparse_july/lefse.in -c 2 -u 1 -o 1000000"
system(format.command)
lefse.command <- paste(paste("~/miniconda2/bin/python ~/lefse/run_lefse.py ",results_folder,"lefse.in " ,results_folder, "lefse.res",sep=""),
"-a", anova.alpha, "-w", wilcoxon.alpha, "-l", lda.cutoff,
"-e", as.numeric(wilcoxon.within.subclass), "-y", as.numeric(one.against.one),
"-s", mult.test.correction)
lefse.command
#"~/miniconda2/bin/python ~/lefse/run_lefse.py ~/Desktop/uparse_july/lefse.in ~/Desktop/uparse_july/lefse.res -a 0.05 -w 0.05 -l 3 -e 1 -y 1 -s 0"
system(lefse.command) #this will print out the number of significant OTUs (make sure it's not 0)
lefse.out <- read.table(paste(results_folder,"lefse.res",sep=""), header = FALSE, sep = "\t")
names(lefse.out)<-c("taxon","log.max.pct","direction","lda","p.value")
(lefse.out<-na.omit(lefse.out))
Palette_LTBI_treatment <- c("#377eb8","#984ea3","pink","blue") #color palette for plots
if(length(unique(lefse.out$direction))<3){
lefse.out$lda[lefse.out$direction==unique(lefse.out$direction)[1]] <-
-1*lefse.out$lda[lefse.out$direction==unique(lefse.out$direction)[1]]
}
lefse.out$taxon<-factor(lefse.out$taxon,levels=lefse.out$taxon[order(lefse.out$lda)])
g1<-ggplot(data=lefse.out,aes(x=taxon,y=lda,color=direction,fill=direction))+
geom_bar(stat="identity")+
coord_flip()+
theme_base()
if(length(unique(lefse.out$direction))<3){
g1<-g1+scale_color_manual(values=c(Palette_LTBI_treatment))+
scale_fill_manual(values=c(Palette_LTBI_treatment))
}
print(g1)
The abscissa shows the linear discriminant analysis (LDA) score for each OTU—a higher score means that the OTU is more important according to LEfSe to discriminate between IGRA+ and HRZE individuals:

ltk<-as.character(lefse.out$taxon)
phy_ra_ltk<-prune_taxa(ltk,phy.lefse)
phy_ra_ltk_m<-psmelt(phy_ra_ltk)
phy_ra_ltk_m$OTU<-factor(phy_ra_ltk_m$OTU, levels=lefse.out$taxon[order(lefse.out$lda)])
g2<-ggplot(phy_ra_ltk_m,aes(x=OTU,
y=Abundance,color=TB_status,
fill=TB_status))+
geom_boxplot(position=position_dodge(),
colour="black", # Use black outlines,
size=.3,alpha=0.5) + # Thinner lines
theme_base()+ xlab("")+ coord_flip() +
scale_y_continuous(limits = c(0,200))
if(length(unique(lefse.out$direction))<3){
g2<-g2+scale_color_manual(values=c(Palette_LTBI_treatment))+
scale_fill_manual(values=c(Palette_LTBI_treatment))
}
print(g2)
Using ggplot's geom_boxplot function, we can plot the most significantly differentially changed OTUs for LTBI vs HRZE people. I left the outliers in just to highlight that they are there, although we removed them for the actual paper for aesthetic purposes:
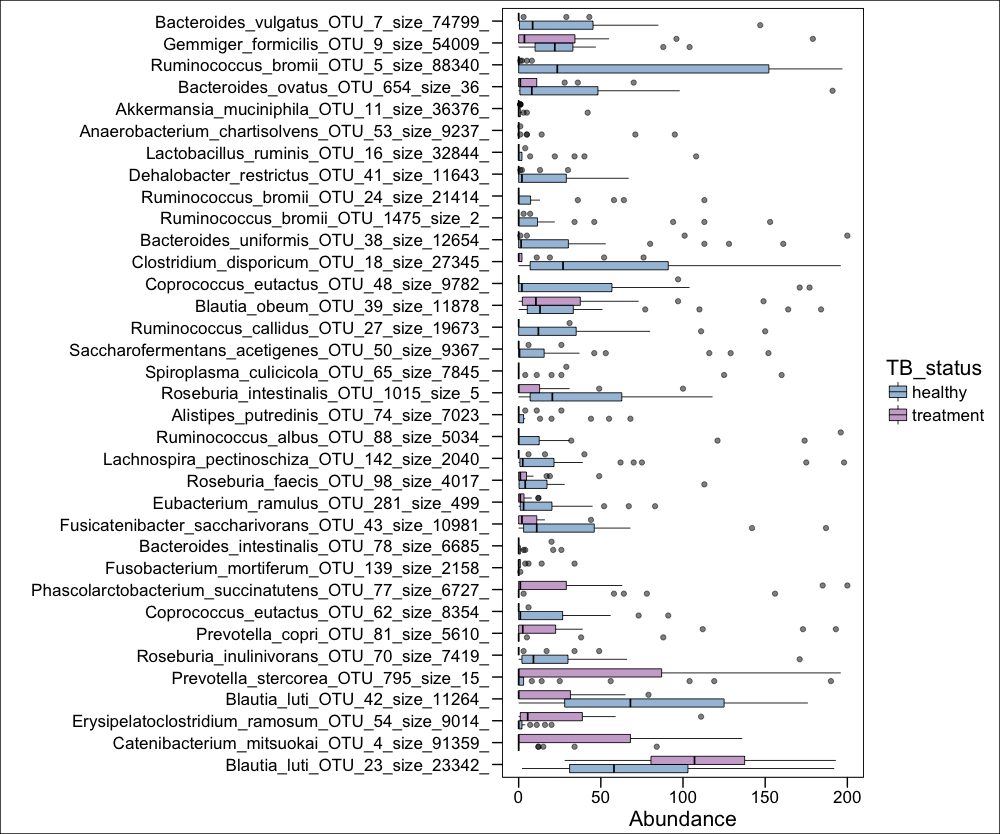 Figure 2D from paper
Figure 2D from paper
Part 4: Principal Coordinate Analysis
phy_ord <- ordinate(phyIGRA_pos_treatment, "NMDS", "bray")
p <- plot_ordination(phyIGRA_pos_treatment,phy_ord, color="TB_status")
p + geom_point(size=4)
Palette_LTBI_treatment <- c("#377eb8","#984ea3") #set the color palette
phy_ord <- ordinate(phyIGRA_pos_treatment, "NMDS", "bray")
p <- plot_ordination(phyIGRA_pos_treatment,phy_ord, color="TB_status")
p + geom_point(size=4) + theme(legend.text = element_text(size=15),
axis.title.x = element_text(size=20),
axis.title.y = element_text(size=20),
axis.text.x = element_text(size=15),
axis.text.y = element_text(size=15)) +
scale_colour_manual(values=Palette_LTBI_treatment)
The Nonmetric Multidimensional Scaling (NMDS) output does not give traditional PCA % variance values, but is good for count data, and performs well separating people on HRZE and healthy LTBI controls:
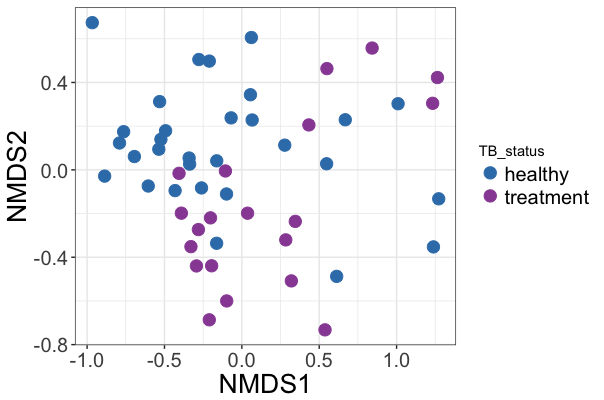 Figure 2A from paper
Figure 2A from paper
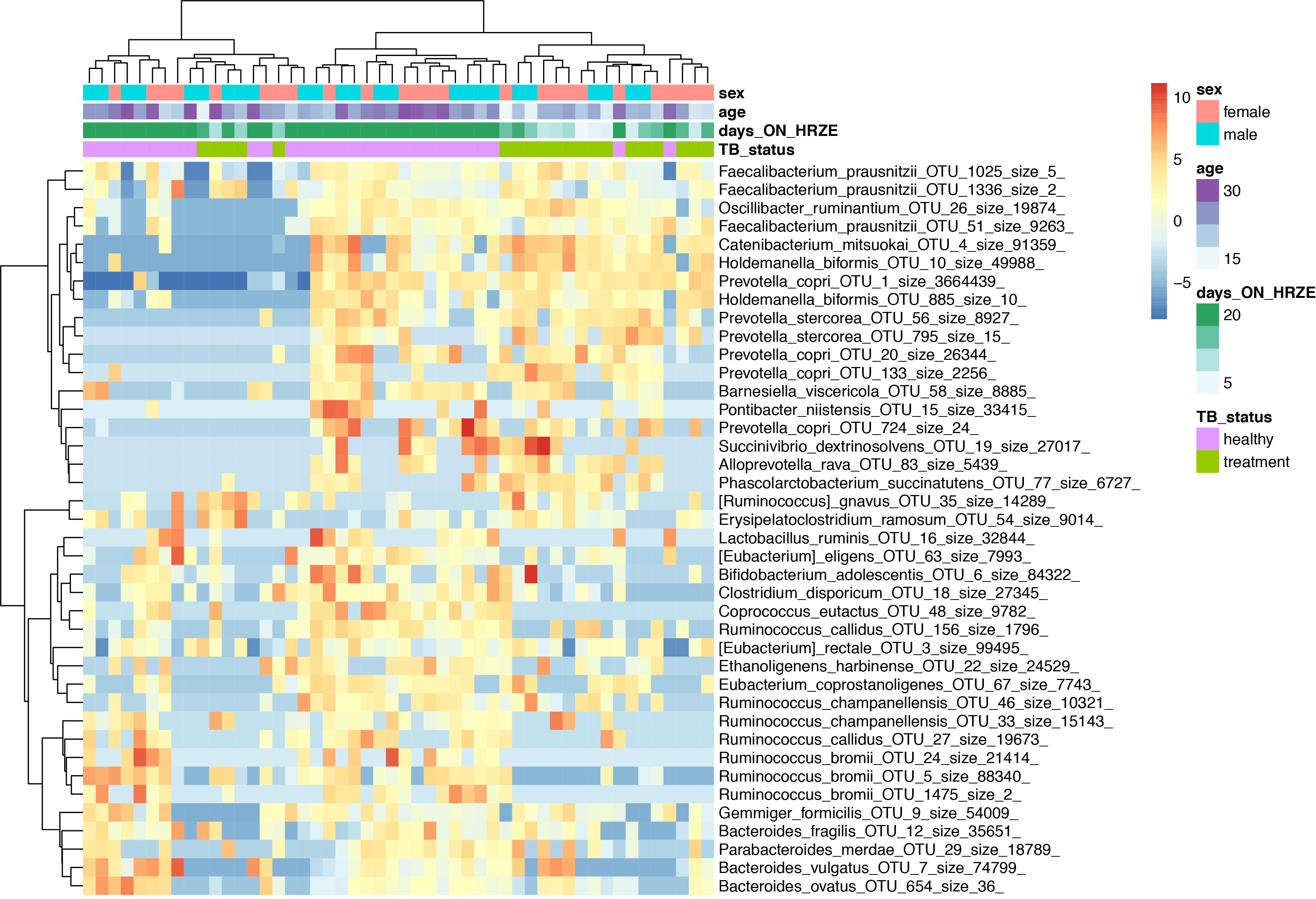
Part 5: Making a heatmap
Heatmaps are my personal favorite way of looking at this type of data. Ideally one would put much more information than just a heatmap into a single figure, but let's start with making a heatmap of just the top 40 most differentially abundant OTUs, and then perform unsupervised hierarchical clustering.
rst <- varianceStabilizingTransformation(dig, blind=FALSE,fitType = "parametric")
sampleDists <- dist( t( assay(rst) ) )
sampleDists
topVarGenes <- head(order(rowVars(assay(rst)),decreasing=TRUE),40)
mat <- assay(rst)[ topVarGenes, ]
mat <- mat - rowMeans(mat)
rst$days_ON_HRZE <- as.numeric(rst$days_ON_HRZE)
df <- as.data.frame(colData(rst)[,c("TB_status","days_ON_HRZE","age","sex")]) #"days_ON_HRZE","TB_status"
pheatmap(mat, annotation_col=df,clustering_distance_rows = "correlation",
clustering_method = "ward.D2",show_colnames = F)
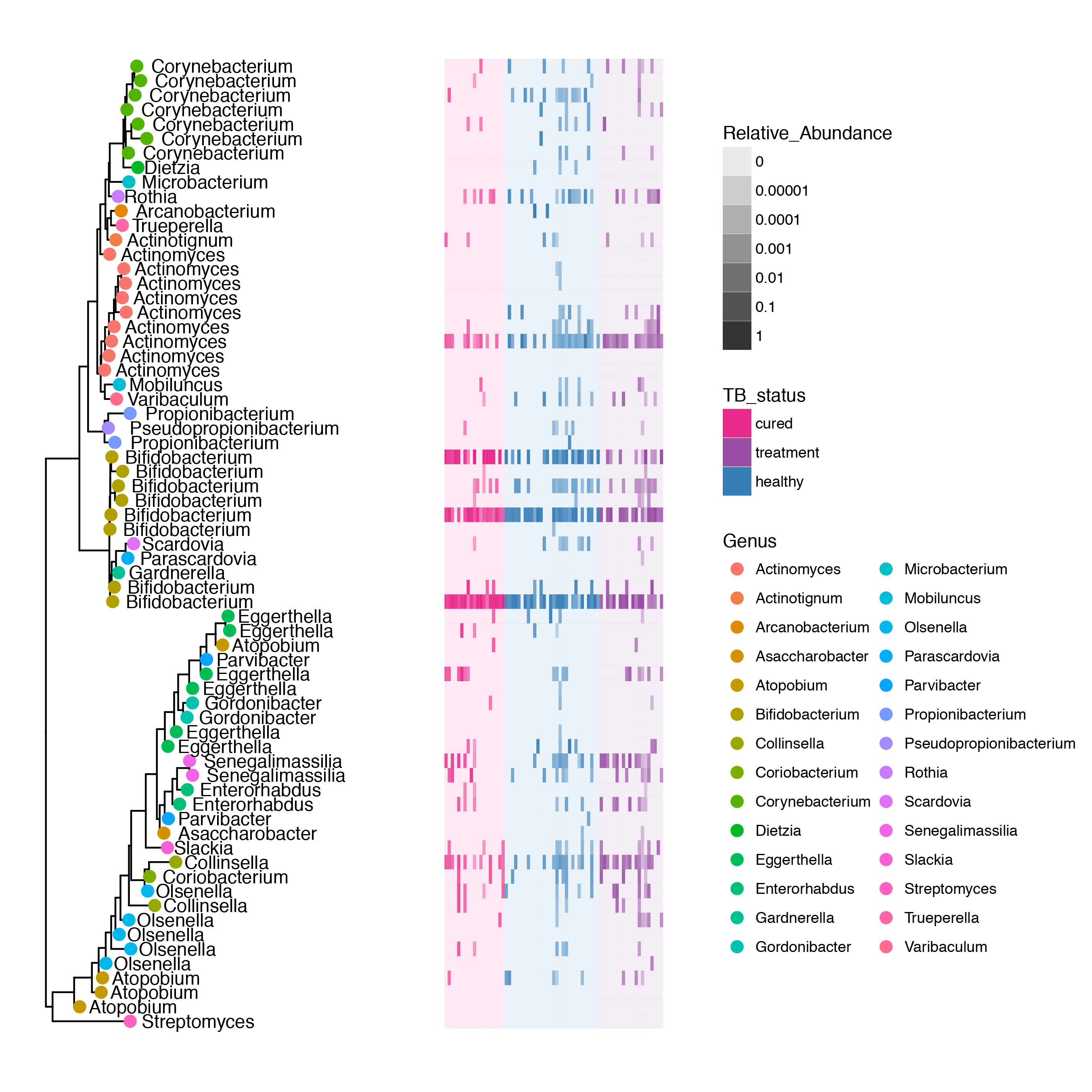
Part 6: Making a phylogenetic tree + heatmap
People always ask me about individuals on treatment (HRZE) and what effect(s) there are on the Actinobacteria in the microbitoa. Interestingly, the major effects are in the Firmicutes, and as a Phylum, the Actinobacteria are largely untouched, with the exception of a species of Bifidobacterium. The plot below takes the phylogenetic tree of Actinobacteria (generated from make_phylogeny.py) and maps the relative abundance of each OTU on the tree in a heatmap.
p_actino <- subset_samples(phy,!is.na(IGRA) & IGRA!="")
p.species <- subset_taxa(p_actino, Phylum=="Actinobacteria")
tr <- phy_tree(p.species)
spec <- as.data.frame(get.tax(p.species))
gt <- ggtree(tr, branch.length = "y") %<+% spec
gd <- gt$data
tt <- get.otu.melt(p.species,filter.zero=FALSE)%>%left_join(select(gd,otu=label,x,y),by="otu") %>%
arrange(TB_status) %>% mutate(sample2=factor(sample,levels=unique(sample)),
col=as.numeric(sample2),x.col=scales::rescale(col,to=c(1.3,2)))
tt$TB_status<-factor(tt$TB_status,levels=c("cured","treatment","healthy"))
Palette_actino <- c("#e7298a","#984ea3","#377eb8")
g1 <- gt + geom_tippoint(data=gd$istip,aes(color=Genus),size=3) +
geom_text(data=gd$istip,aes(label=Genus,x=x+0.001),hjust=-0.1,check_overlap = F) +
geom_tile(data=tt,aes(x=x.col,y=y,fill=TB_status,alpha=pctseqs),position="dodge") +
scale_alpha_continuous(trans=log_epsilon_trans(0.00001),aes(show.legend=Relative_Abundance)) +
theme(legend.position="right")
g1 + scale_fill_manual(values=Palette_actino)